Cleaning and Sanitization in the Cosmetics Industry
Maintaining cleanliness in a cosmetic manufacturing unit is not just about looking clean — it’s about making sure every product we make is safe, pure, and high quality.
Cleaning and sanitization are both essential parts of Good Manufacturing Practices (GMP) in the cosmetics industry.
Let’s understand what they are, why they are important, and how we do them step by step.
🧴 1. What is Cleaning?
Cleaning means removing dirt, dust, oil, leftover product, or any visible or invisible residue from surfaces, equipment, or areas.
It helps remove all unwanted materials before sanitization starts.
Why Cleaning is Important
It keeps equipment and work areas free from contamination.
It avoids product mix-ups and cross-contamination.
It helps maintain product quality and consistency.
It’s a key requirement under GMP.
🧽 2. Types of Cleaning in Cosmetics Industry
Cleaning is mainly divided into two types: A-type cleaning and B-type cleaning.
✅ A-Type Cleaning (Product Changeover Cleaning)
Done when you change from one product to another (for example, from shampoo to lotion).
It’s a deep cleaning process that removes all traces of the old product.
It includes cleaning all contact surfaces — tanks, mixers, transfer lines, and filling machines.
Usually done after every batch or product change.
✅ B-Type Cleaning (Routine or Daily Cleaning)
Done daily or at the end of each shift.
Keeps the production area and equipment clean while making the same product.
Includes cleaning of tables, external parts of machines, floors, and walls.
Helps in preventing dust or microbial build-up.
🧼 3. Cleaning Process Step-by-Step
Let’s look at the typical cleaning process used in cosmetic manufacturing:
Step 1: Pre-Cleaning
Remove leftover or bulk product manually using brushes, cloths, or scrapers.
Makes the next cleaning steps easier and faster.
Step 2: Rinse with Water
Wash all parts with warm or purified water to remove loose particles or product residues.
Step 3: Apply Cleaning Agent or Detergent
Use an approved cleaning solution (alkaline or surfactant-based).
Apply using brushes, sponges, or circulation systems (for tanks and pipes).
Allow enough time for the solution to work on the dirt or oil.
Step 4: Rinsing
Rinse properly with purified or deionized water to remove all detergent traces.
This step is very important — leftover detergent can affect the next product batch.
Step 5: Drying
Dry all cleaned surfaces completely using hot air, lint-free cloths, or natural air drying.
Moisture can cause microbial growth, so drying is a must.
Step 6: Inspection
Visually check the equipment and area.
If clean, sign and record in the cleaning logbook.
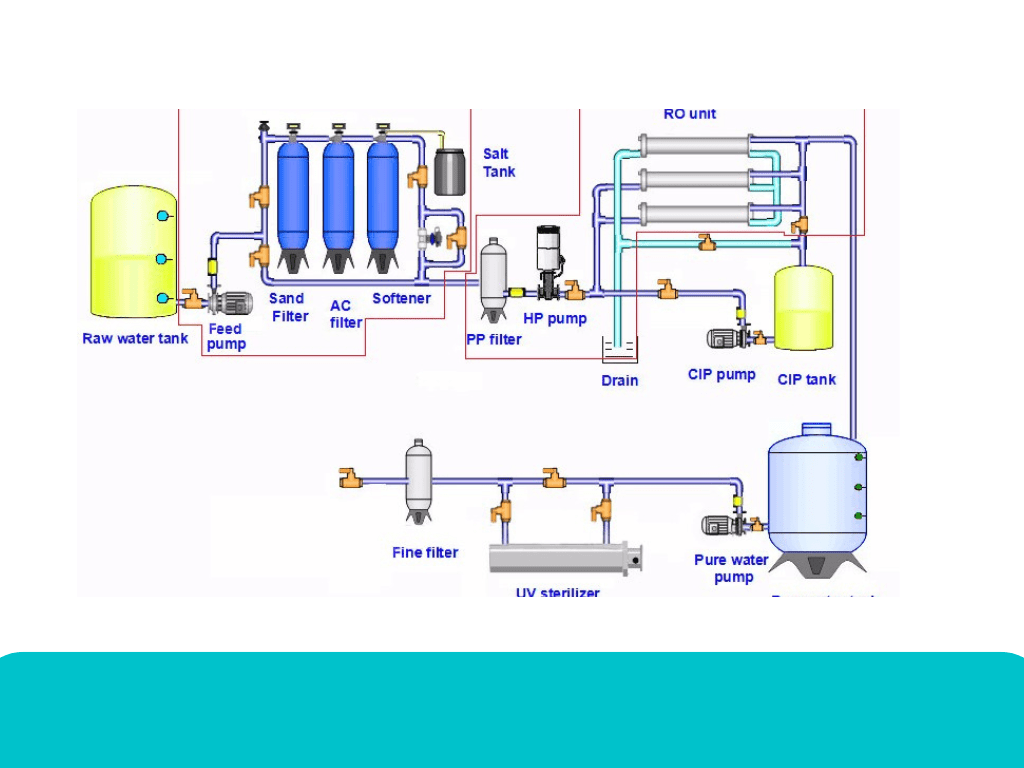
⚙️ 4. Equipment Cleaning Under GMP
In the cosmetics industry, equipment like mixers, tanks, filling machines, filters, and hoses must be cleaned as per written SOPs (Standard Operating Procedures).
Main Points to Remember
1. Validation:
Cleaning methods must be tested to show that they effectively remove residues.
2. Dedicated vs. Multi-Use Equipment:
Dedicated equipment (used for one product only) needs less frequent cleaning.
Multi-use equipment must be cleaned deeply every time a product changes.
3. CIP (Clean-In-Place):
For large tanks and pipelines.
Cleaning happens automatically by circulating cleaning and rinsing liquids inside without dismantling.
4. Manual Cleaning:
Used for small parts or tools.
Operators follow proper SOPs and use approved brushes and cleaning agents.
💧 5. What is Rinse Sampling?
Rinse sampling is a test done after cleaning to confirm that equipment is clean.
How It Works
Collect the final rinse water from the cleaned equipment.
The sample is tested for:
pH
Conductivity
Microbial count
Residual detergent traces
If all values are within acceptable limits, the equipment is marked as CLEAN and READY TO USE.
🧫 6. What is Swab Sampling?
Sometimes, instead of rinse water, we test the surface directly using a swab.
Swab Sampling Process
1. Take a sterile swab (cotton or polyester).
2. Moisten it with purified water or solvent.
3. Wipe a fixed area (like 25 cm²) of the equipment surface.
4. Put the swab into a sterile tube and send it for testing.
Purpose
To find out if any residue, detergent, or microorganisms are still on the surface.
It gives direct proof that cleaning was effective.
🧰 7. Why Cleaning is Important in GMP
Under GMP (Good Manufacturing Practices), cleaning is a must because it:
Prevents contamination between different batches.
Protects product quality and safety.
Reduces risk of product recall or rejection.
Fulfills regulatory requirements (like ISO 22716, FDA, BIS).
Keeps the environment safe for workers.
Simply put — cleaning is the backbone of quality in cosmetics manufacturing.
🌿 8. What is Sanitization?
Sanitization is the process of killing or reducing microorganisms on equipment, surfaces, and rooms to safe levels.
It is done after cleaning — because cleaning removes dirt, but sanitization kills the germs that might still remain.
🧴 9. Sanitization Process Step-by-Step
Step 1: Choose Sanitizing Agent
Use only approved sanitizers, such as:
70% Isopropyl Alcohol (IPA)
Hydrogen Peroxide (3–6%)
Sodium Hypochlorite (bleach)
Quaternary Ammonium Compounds (QAC)
Peracetic Acid
Step 2: Prepare Solution
Prepare the sanitizer as per required concentration.
Label the container with date, strength, and expiry.
Step 3: Apply Sanitizer
Spray the solution on all surfaces, equipment, and areas.
You can also use mops, wipes, or fogging machines.
Step 4: Contact Time
Leave it for 10–15 minutes to allow full germ-kill effect.
Do not wipe it off immediately.
Step 5: Drying
Let the surface air dry.
Avoid touching it until dry.
📅 10. How Often to Sanitize?
Production Areas: Daily or after every shift
Filling Room: Before and after every batch
Storage Room: Weekly or as per SOP
Clean Room: As per schedule (daily or alternate day)
🌼 11. Benefits of Sanitization
Kills harmful microorganisms
Prevents contamination in products
Extends product shelf life
Helps meet GMP and ISO 22716 standards
Reduces batch rejection or recall chances
Keeps production environment hygienic and safe
📋 12. Cleaning & Sanitization Records
Every cleaning and sanitization activity must be properly documented.
Documentation helps in traceability and regulatory inspections.
Important Records
Cleaning Logbooks
Equipment Cleaning Records
Sanitization Schedule
Swab and Rinse Test Reports
Cleaning Validation Reports
Operator Training Records
Proper documentation = proof of compliance.
🧠 13. Summary
Process Purpose Frequency Validation
A-Type Cleaning Deep cleaning during product change After each product/batch Yes
B-Type Cleaning Daily maintenance cleaning Daily or shift-end Yes
Rinse Sampling Test of final rinse water After cleaning Yes
Swab Sampling Direct surface test After cleaning Yes
Sanitization Kill microorganisms Daily or weekly Yes
🌟 14. Conclusion
Cleaning and sanitization are not just regular tasks — they are the heart of quality control in the cosmetics industry.
Without proper cleaning, even the best ingredients can turn unsafe.
A disciplined cleaning and sanitization program ensures every product that reaches the customer is pure, safe, and trustworthy.