Corrective and Preventive Action (CAPA) is a critical component of quality management systems (QMS) used across various industries, including cosmetics, to ensure product safety, quality, and regulatory compliance. In the cosmetics industry, CAPA refers to a systematic approach to identifying, investigating, and resolving quality issues while preventing their recurrence or occurrence. It involves two key elements: Corrective Action, which addresses existing problems, and Preventive Action, which proactively mitigates potential issues. CAPA ensures that cosmetic products meet consumer expectations, adhere to regulatory standards, and maintain brand reputation.
In the cosmetics industry, where consumer safety, product efficacy, and regulatory compliance are paramount, CAPA plays a vital role in addressing issues like contamination, incorrect labeling, defective packaging, or non-compliance with standards such as Good Manufacturing Practices (GMP) or regulations set by bodies like the FDA, EU Cosmetics Regulation, or ISO 22716. By implementing CAPA, cosmetic manufacturers can enhance product quality, reduce risks, and foster a culture of continuous improvement
CAPA in the Cosmetics Industry: A Comprehensive Guide to Quality Management
The cosmetics industry thrives on delivering safe, effective, and high-quality products to consumers. However, issues like product contamination, mislabeling, or packaging defects can jeopardize consumer trust and regulatory compliance. This is where Corrective and Preventive Action (CAPA) comes into play. CAPA is a cornerstone of quality management systems (QMS) that helps cosmetic manufacturers identify, resolve, and prevent quality issues. In this blog, we’ll explore what CAPA is, its importance in the cosmetics industry, the CAPA process, and best practices for implementation, all tailored to ensure compliance and consumer satisfaction.
What is CAPA?
CAPA stands for Corrective and Preventive Action, a structured methodology used to address quality issues and prevent their recurrence or occurrence. In the cosmetics industry, CAPA is integral to maintaining product safety, efficacy, and compliance with regulations like the FDA’s 21 CFR Part 820, ISO 22716 (Cosmetics GMP), and the EU Cosmetics Regulation (EC No 1223/2009).
Corrective Action: Focuses on identifying the root cause of an existing issue (e.g., a batch of contaminated lipstick) and implementing measures to eliminate it, ensuring the problem does not recur.
Preventive Action: Proactively identifies potential risks (e.g., a packaging machine prone to errors) and implements measures to prevent issues before they arise.
CAPA is not just about fixing problems; it’s about building a robust quality system that ensures consistent product quality and regulatory adherence.
Why is CAPA Important in the Cosmetics Industry?
The cosmetics industry faces unique challenges, from ensuring ingredient safety to meeting stringent regulatory requirements. CAPA is critical for several reasons:
Consumer Safety: Cosmetics are applied directly to the skin, eyes, or hair, making safety a top priority. CAPA helps address issues like microbial contamination or harmful ingredients, protecting consumers from adverse effects.
Regulatory Compliance: Regulatory bodies like the FDA and EU require cosmetic manufacturers to have systems in place to address non-conformities. Failure to implement CAPA can result in warning letters, product recalls, or fines.
Brand Reputation: A single quality issue, such as mislabeled products or allergic reactions, can damage a brand’s reputation. CAPA ensures issues are resolved swiftly and prevented in the future.
Cost Efficiency: By addressing root causes and preventing recurrence, CAPA reduces costs associated with recalls, rework, and customer complaints.
Continuous Improvement: CAPA fosters a culture of quality, encouraging manufacturers to analyze trends, improve processes, and enhance product reliability.
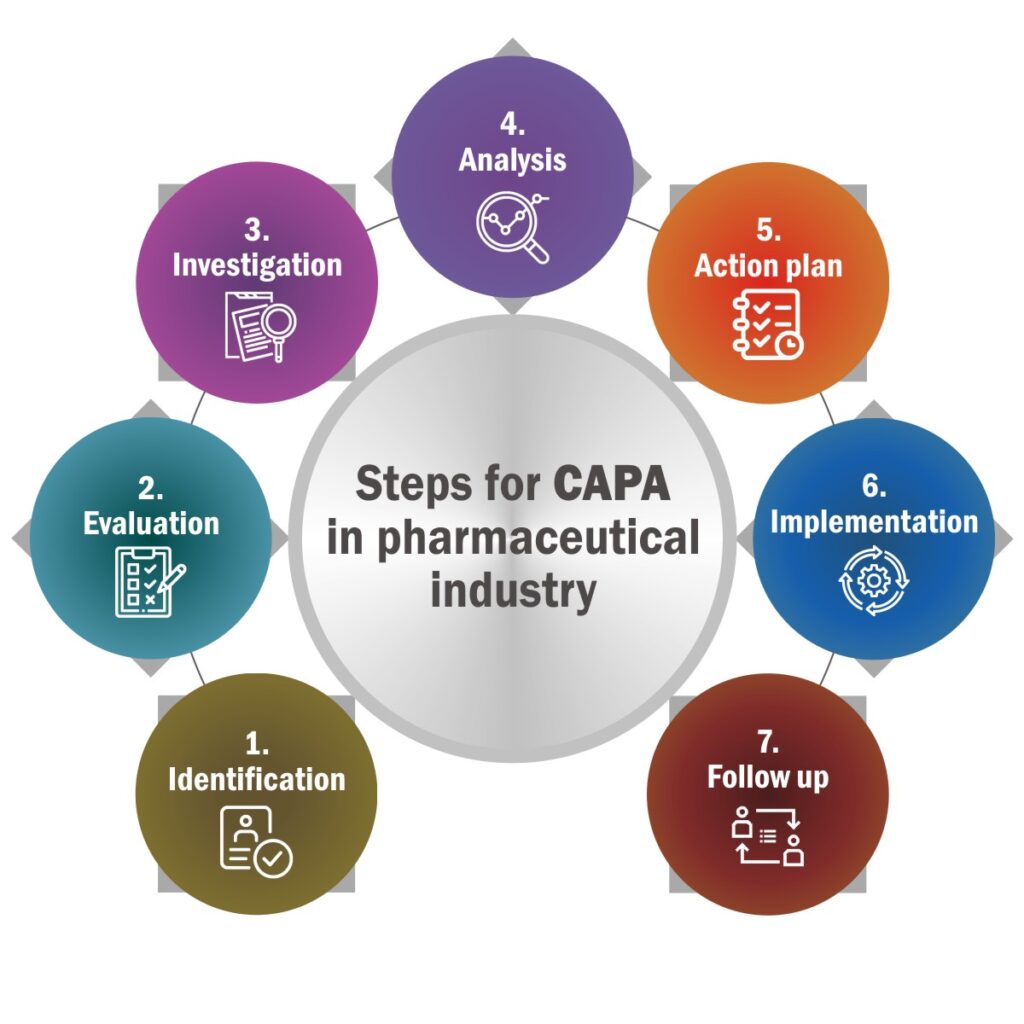
The CAPA Process in the Cosmetics Industry
The CAPA process is a systematic, step-by-step approach to managing quality issues. Below are the key steps tailored to the cosmetics industry:
1. Identify the Issue
The first step is to detect and document any quality issue or non-conformity. Issues in cosmetics can arise from various sources, such as:
Customer complaints (e.g., skin irritation from a moisturizer).
Internal audits revealing deviations from GMP standards.
Production issues, like inconsistent batch mixing or defective packaging.
Regulatory inspections identifying non-compliance.
Example: A customer reports that a batch of foundation causes skin redness. The issue is documented with details like batch number, date, and customer feedback.
2. Contain the Problem
Immediate action is taken to limit the impact of the issue. This may involve:
Quarantining affected products.
Halting production or distribution.
Notifying retailers or consumers.
Example: The manufacturer quarantines all foundation batches from the same production line and pauses distribution until the issue is investigated.
3. Perform/SD-3 Conduct Root Cause Analysis
Root cause analysis (RCA) identifies the underlying cause of the issue. Common tools include:
5 Whys: Asking “why” repeatedly to drill down to the root cause.
Fishbone Diagram: Categorizing causes (e.g., materials, processes, equipment).
Pareto Analysis: Prioritizing the most significant causes.
Example: RCA reveals that the foundation’s redness was caused by an unlisted allergen in a new ingredient batch.
4. Develop Corrective Actions
Corrective actions address the root cause to eliminate the issue. These actions should be:
Specific: Clearly defined (e.g., update ingredient sourcing protocols).
Measurable: Trackable via metrics or audits.
Achievable: Feasible within resources and timelines.
Relevant: Directly tied to the root cause.
Time-bound: Completed within a set deadline.
Example: The manufacturer revises supplier quality checks to ensure allergen-free ingredients.
5. Implement Corrective Actions
Execute the corrective actions, ensuring proper communication and training for staff. Track progress to avoid delays.
Example: The supplier quality team is trained on new allergen detection protocols, and checks are implemented immediately.
6. Develop Preventive Actions
Preventive actions address potential risks to avoid similar issues elsewhere. This may include:
Process improvements (e.g., automated quality checks).
Staff training on new procedures.
Equipment upgrades to prevent errors.
Example: The manufacturer installs allergen detection sensors on all production lines.
7. Verify Effectiveness
Confirm that corrective and preventive actions have resolved the issue and prevented recurrence. This involves:
Follow-up audits.
Testing (e.g., product safety tests).
Monitoring customer feedback.
Example: Post-implementation testing shows no further allergen complaints, and audits confirm compliance.
8. Document and Close the CAPA
Thorough documentation is essential for compliance and future reference. Include:
Issue description and evidence.
Root cause findings.
Action plans and outcomes.
Effectiveness verification results.
Example: The CAPA report is archived in the QMS, and the case is closed after QA approval.
https://cosmeticgyaan.com/what-is-gmp-good-manufacturing-practices/
Best Practices for CAPA in Cosmetics
To ensure an effective CAPA system in the cosmetics industry, follow these best practices:
Use CAPA Software: Tools like MasterControl or Qualio streamline documentation, workflows, and trend analysis, ensuring compliance with ISO 22716 and FDA regulations.Train Employees: Regular training on CAPA processes fosters a quality-driven culture. Employees should know how to report issues and contribute to solutions.
Integrate with QMS: CAPA should align with other QMS processes, such as complaint handling and risk management, for a holistic approach.
Conduct Regular Audits: Proactive audits help identify potential issues before they escalate, reducing the need for corrective actions.
Leverage Data: Analyze trends from customer feedback, production data, and audits to inform preventive actions.
Engage Cross-Functional Teams: Involve quality assurance, production, and regulatory teams to ensure comprehensive problem-solving.
Real-World Example: CAPA in Action
A cosmetic company discovers that a batch of mascara causes eye irritation due to improper mixing of preservatives. Here’s how CAPA is applied:
Identification: Customer complaints trigger an investigation.
Containment: The affected batch is recalled, and production is paused.
Root Cause Analysis: The 5 Whys reveals a calibration error in the mixing equipment.
Corrective Action: The equipment is recalibrated, and operators are retrained.
Preventive Action: Automated calibration checks are added to all mixing machines.
Verification: Post-implementation testing confirms no further irritation complaints.
Documentation: The CAPA report is filed, detailing all steps and outcomes.
This process resolves the issue, prevents recurrence, and ensures compliance with GMP standards.
Challenges and Solutions
Implementing CAPA in the cosmetics industry can be challenging. Common issues include:
Resource Constraints: Small manufacturers may lack resources for robust CAPA systems. Solution: Use cost-effective CAPA software to automate processes.
Complex Investigations: Identifying root causes can be time-consuming. Solution: Train staff on RCA tools like Fishbone Diagrams and 5 Whys.
Documentation Burden: Extensive record-keeping can overwhelm teams. Solution: Adopt electronic QMS platforms for efficient documentation.
Conclusion
CAPA is a vital tool for ensuring quality, safety, and compliance in the cosmetics industry. By systematically addressing existing issues and preventing future ones, CAPA helps manufacturers deliver safe, effective products while meeting regulatory requirements. A well-executed CAPA process enhances operational efficiency, reduces costs, and builds consumer trust. By following the steps outlined—identification, containment, root cause analysis, corrective and preventive actions, verification, and documentation—cosmetic companies can create a culture of continuous improvement.
To optimize CAPA, invest in training, leverage technology, and integrate it with your QMS. Whether you’re addressing a contamination issue or preventing packaging errors, CAPA empowers you to maintain high standards and stay competitive in the fast-paced cosmetics market.